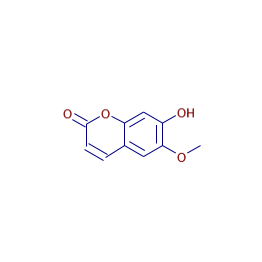
Scopoletin
970
Reference
Systematic / IUPAC Name: 7-Hydroxy-6-methoxy-2H-chromen-2-one
ID: Reference970
Other Names:
6-Methylesculetin;
7-Hydroxy-6-methoxycoumarin;
6-O-Methylesculetin;
6-Methoxyumbelliferone;
6-Methoxy-7-hydroxycoumarin
; more
Formula: C10H8O4
Class: Endogenous Metabolites
Spectral Data
Scopoletin mass spectral data can be found in a separate interface. The data are manually curated and of the highest quality.
Available MS Data
| Used Instruments | LTQ Orbitrap XL; Orbitrap Elite |
| No. of Spectral Trees | 3 |
| No. of Spectra | 1028 |
| Tandem Spectra | MS1, MS2, MS3, MS4, MS5 |
| Ionization Methods | ESI; NSI |
| Analyzers | IT; FT |
| Last Modification | 7/31/2014 3:55:40 PM |
Identificators
| InChI | InChI=1S/C10H8O4/c1-13-9-4-6-2-3-10(12)14-8(6)5-7(9)11/h2-5,11H,1H3 |
| InChI Key | RODXRVNMMDRFIK-UHFFFAOYSA-N |
| Canonical SMILES | COC1=C(C=C2C(=C1)C=CC(=O)O2)O |
| CAS | 92615 |
| Splash | |
| Other Names |
6-Methylesculetin; 7-Hydroxy-6-methoxycoumarin; 6-O-Methylesculetin; 6-Methoxyumbelliferone; 6-Methoxy-7-hydroxycoumarin; 7-Hydroxy-6-methoxy-2H-1-benzopyran-2-one; β-Methylesculetin; Coumarin, 7-hydroxy-6-methoxy-; 7-Hydroxy-6-methoxy-2H-chromen-2-one; 2H-1-Benzopyran-2-one, 7-hydroxy-6-methoxy-; Esculetin-6-methyl ether; 7-Hydroxy-5-methoxycoumarin; Gelseminic acid; Chrysatropic acid; Murrayetin; Scopoletine; Scopoletol; Escopoletin |
In Other Databases
| Wikipedia | Scopoletin |
| ChemSpider | 4444113 |
| ChemIDPlus | 000092615 |
| HMDb | HMDB34344 |
| PubChem | 5280460 |
| ChEMBL | CHEMBL71851 |
| ChEBI | CHEBI:17488 |
| KEGG | C01752 |